Molecular imaging collaborations

Noninvasive imaging of biological processes
The Molecular Imaging Lab helps develop integrated imaging approaches to noninvasively monitor and track biological processes. These include peripheral and myocardial angiogenesis, vascular remodeling, atherosclerosis, and cancer using molecular, physiological, functional, and anatomical imaging modalities including nuclear (SPECT/PET), X-ray CT, and in vivo optical imaging.
Noninvasive imaging of biological processes
The Molecular Imaging Lab helps develop integrated imaging approaches to noninvasively monitor and track biological processes. These include peripheral and myocardial angiogenesis, vascular remodeling, atherosclerosis, and cancer using molecular, physiological, functional, and anatomical imaging modalities including nuclear (SPECT/PET), X-ray CT, and in vivo optical imaging.
Such imaging strategies will eventually lead to individualized programs for disease prevention through advanced diagnosis, risk stratification, and targeted cellular and genetic therapies. This will result in more successful and efficient health care.
Since the time of Galileo, imaging has been the eyes of science. Centuries later, the National Academy of Engineering selected imaging as one of the most outstanding engineering achievements of the 20th century.
The combination of different imaging modalities and technologies for mapping biomolecular and biological processes within a single cell or whole organs has an extraordinary potential for revolutionizing the diagnosis and treatment of pathophysiological disorders. As a result, it has reduced the social and economic costs associated with the clinical management of diseases.
Research conducted through the Molecular Imaging Lab develops integrated imaging approaches, which will eventually lead to individualized programs for disease prevention through advanced diagnosis, risk stratification, and targeted cell therapies. It will result in more successful and efficient health care.
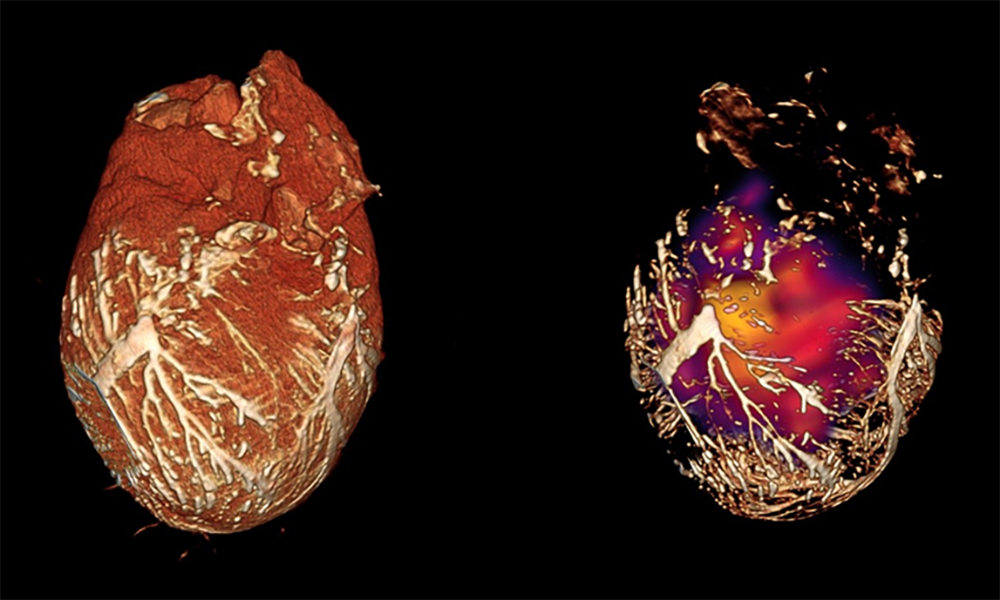
Theme 1: Theme One: Noninvasive monitoring of therapeutic angiogenesis and arteriogenesis
Angiogenesis, defined as the formation of new blood vessels, is critical in the common pathological states such as cancer, myocardial ischemia, or coronary and peripheral arterial disease.
Theme 1: Theme One: Noninvasive monitoring of therapeutic angiogenesis and arteriogenesis
Angiogenesis, defined as the formation of new blood vessels, is critical in the common pathological states such as cancer, myocardial ischemia, or coronary and peripheral arterial disease.
Top academic institutions around the world are pursuing therapeutic strategies directed at the stimulation of angiogenesis using gene therapy or stem cells. However, despite positive results in animal studies, clinical trials of stimulated angiogenesis in CAD and PAD demonstrated no clear benefit over placebo.
The area of study to develop new noninvasive imaging approaches to tracking biological processes with the potential to translate to clinical practice has been largely underexplored.
Our studies focus on developing advanced multimodal imaging strategies to noninvasively follow both angiogenic processes and the migration of stem cells to the area of injury. We also examine molecular targets required in the formation and maturation of vessels with the potential to be used as targeted tracers to monitor therapeutic interventions.
Previously, we demonstrated the feasibility of the intramyocardial insulin-like growth factor gene transfer after myocardial infarction to promote sustained angiogenesis, which improved myocardial function.
Similar studies are performed with cell-based approaches, including transplantation of stem cells or cell-seeded bioengineered scaffolds. Current studies funded by the American Heart Association focus on the development, radiosynthesis, and characterization of RGD-peptide constructs, which are used to non-invasively track angiogenic processes in the post-infarct myocardium in the onset of diabetes.
We also focus on developing imaging strategies to monitor therapeutic angiogenesis using novel multimodal probes such as multimeric RGD-containing peptide constructs and assess the efficacy of novel therapies, including nanoparticle drug-delivery systems transplanted cell homing and survival using reporter gene imaging techniques.
Theme 2: Theme Two: Developing novel multimodal multifunctional targeted tracers
With the advent of molecular probes, imaging enables the determination of both the temporal and the spatial distributions of biological processes.
Theme 2: Theme Two: Developing novel multimodal multifunctional targeted tracers
With the advent of molecular probes, imaging enables the determination of both the temporal and the spatial distributions of biological processes.
The development of hybridized systems requires the availability of novel multimodal and multifunctional imaging agents, which can describe biology in vivo on a single component level and on the level of system processes and mechanisms, which can be further used for in silico simulations.
The combination of imaging biological processes and individualized therapies will ultimately lead to the development of novel modular, multifunctional nanoparticle constructs that would allow sustained delivery of targeted therapy linked with multimodality imaging.
Our previous work focused on developing multimodal dendrimer-based probes for blood pool imaging led to a design of modular elements that can be manipulated to create an optimized multifunctional platform. These modular elements consist of liposomes for encapsulation, biodegradable solid particles for sustained drug delivery, and dendrimer nanoparticles or quantum dots for multimodal labeling.
If successful, this multifunctional approach will result in a significant paradigm change and allow the development of an entirely new diagnostic and therapeutic system to evaluate and manage patients.

Theme 3: Co-registration and quantification of multimodal images
The introduction of genetic and cell-based therapies and nanoparticle drug-delivery systems to treat various diseases through the modulation of biological systems has motivated the development of noninvasive imaging approaches to evaluate and track the response to these therapies.
Theme 3: Co-registration and quantification of multimodal images
The introduction of genetic and cell-based therapies and nanoparticle drug-delivery systems to treat various diseases through the modulation of biological systems has motivated the development of noninvasive imaging approaches to evaluate and track the response to these therapies.
Current studies of the angiogenic process require the euthanasia of animals at selected time points because of highly invasive measures or tissue analyses.
We hypothesized that targeted molecular images of virtually any radiotracer uptake could be more accurately quantified by using a high-resolution hybrid nuclear imaging system, such as SPECT-CT or PET-CT, that would provide anatomic localization of the targeted radiotracer within the tissues.
This analysis approach to quantify targeted SPECT images in hindlimbs is now freely available online as a user-friendly tool developed by our collaborators at Yale University.
We also develop tools to quantify molecularly targeted images in the heart using volumes-of-interest defined in X-ray contrast CT images. This approach will allow for possible attenuation and partial-volume error correction of microSPECT and microPET images.
This research provided a strong foundation for a research project in collaboration with the development of molecular probes and applications for novel PET, and SPECT inserts that allow for simultaneous acquisition of functional, targeted nuclear images in the presence of strong magnetic fields within typical magnetic fields resonance scanners.
This research will ultimately lead to the development of novel hybridized imaging modalities that allow for PET/SPECT-MR imaging of biological systems using specialized multimodal multifunctional probes.
BIOMEDICAL IMAGING CENTER
1215 Beckman Institute, 405 N. Mathews Ave., Urbana, Illinois 61801
217-244-0600